How to dilute liquid aromas and nicotine bases
HOW TO DILUTE LIQUIDS BASES AROMAS AND NICOTINE
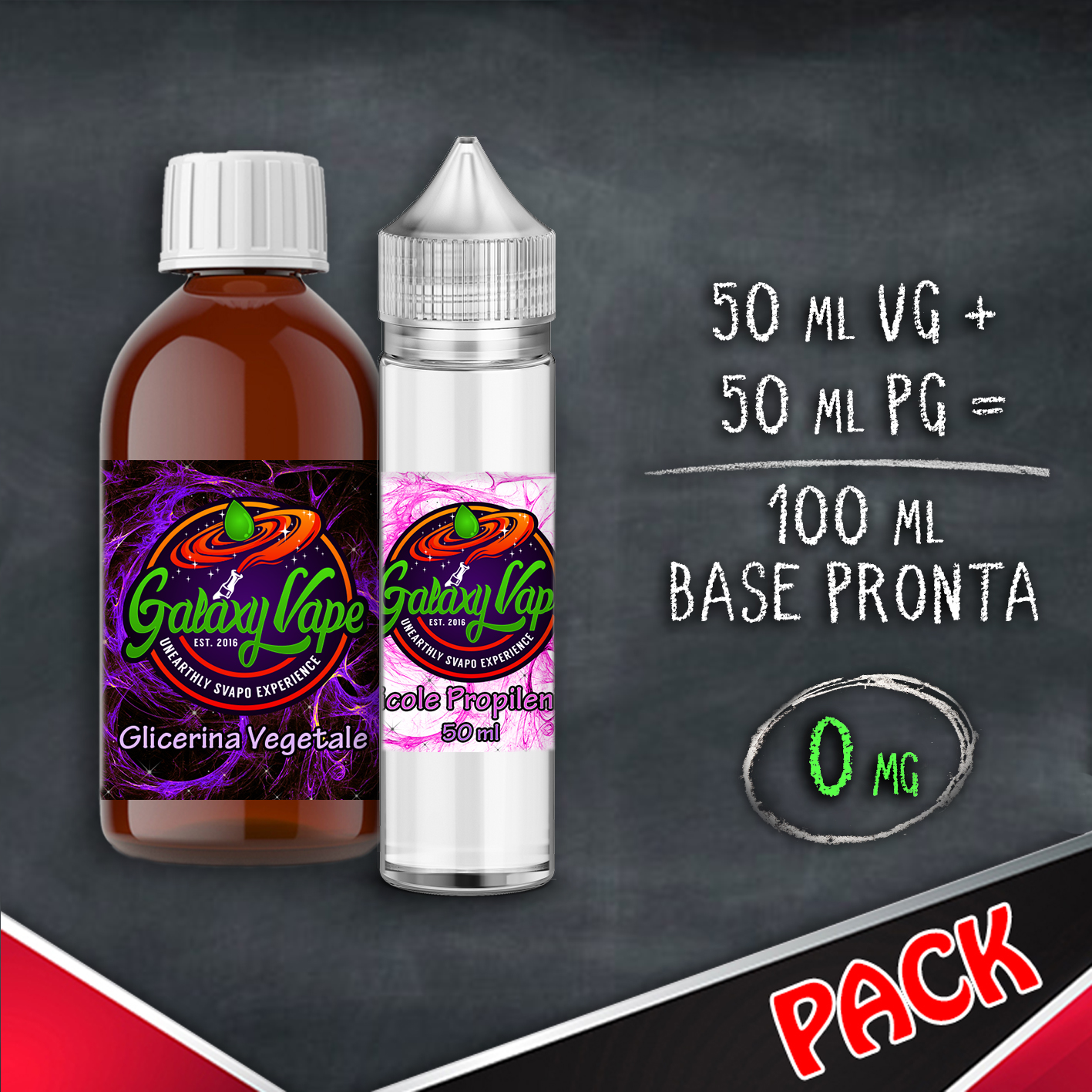
How to dilute liquids aromatic and nicotine bases The constituents of the e-liquid can be mixed in variable percentages according to personal taste. For example, a base consisting of 50% glycerol, 40% glycol and 10% water could be used, and a small part of aromas concentrated in the proportion of 1:10 could be added to the overall base thus obtained; however, it is not uncommon for less components to be used, even only 2 constituents and rarely even one (typically 100% Glycerol).
propylene glycol or E1520 an edible drinkable and injectable food and pharmaceutical additive, used to lengthen or dissolve other substances, is widely used due to the absolute absence of carcinogenicity or genotoxicity demonstrated worldwide by numerous studies [3],
glycerol, E422, a natural constituent of oils and fats naturally present in the human body, is an edible and injectable food and pharmaceutical plant additive, usable in the medical field as a laxative or anti hypertension, in the cosmetic field for creams and soaps for its moisturizing properties and emollients, in the culinary field at the base of cakes and confectioners
food flavorings, i.e. concentrated natural flavors and odors derived from natural and artificial sources, identified by European legislation as natural flavors extracted from natural products and ingredients, are a wide range of molecules such as: limonene (citrus), menthol (mint), pinene (conifers), eucalyptol (eucalyptus), gamma-nonalactone (coconut), frambinone (raspberry), cinnamate (cherry, cinnamon), linalool (basil), geraniol (geranium), vanillin (vanilla), gamma-undecalactone (peach) beta -ion (purple), etc.
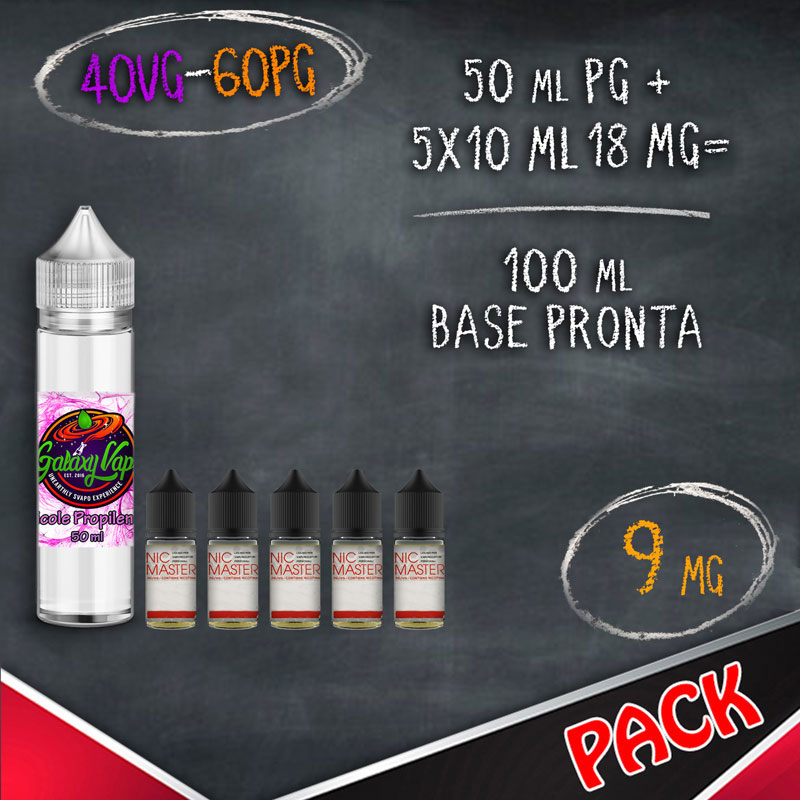
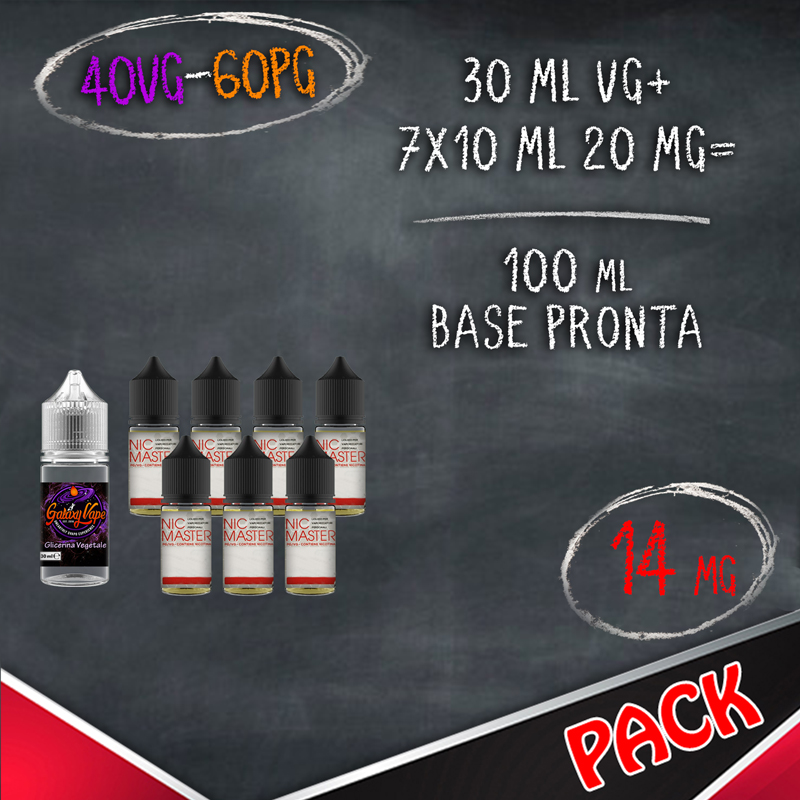
In the table below you will find information for the preparation of your vaping bases where for NICOTINE BASE we indicate the 10 ml bases with nicotine and for LIQUID QUANTITY and FINAL NICOTINE we mean the final result of mixing products.
THE BASE RATIO 50/50, 70/30 AND 80/20 is nothing but the percentage of the two components glycol and glycerin, therefore by mixing half glycol and half glycerine we will obtain a 50/50 base, instead mixing 70% glycerine and 30% glycol we will get a base 70/30 VG / PG.
DO-IT-YOURSELF LIQUID PREPARATION TABLE
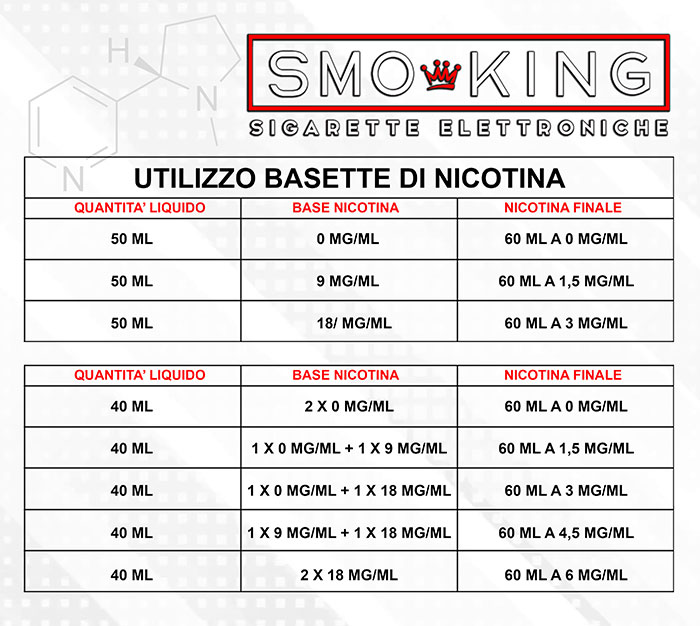
BASES FORMAT 10 ML WITH NICOTINE (BASETTE)
BASETTE TPD 10 ML NICOTINA
GLICOLE E GLICERINA IN KIT
Esempi di diluizione base per la preparazione dei liquidi già inseriti in appositi kit svapo
Galaxy Vape Kit Base Neutra 50/50 100 ml
Pack Base Neutra Galaxy Vape 500 ml
Pack Base Neutra Galaxy Vape 1 L
GLICOLE E GLICERINA IN KIT CON NICOTINA
Base Neutra 250ml 50/50 4mg Nicotina
Base Neutra 200ml 60/40 6mg Nicotina
Base Neutra 100ml 60/40 9mg Nicotina
Base Neutra 100ml 40/60 14mg Nicotina
AROMA PREPARATION
TRIPLE CONCENTRATION AROMA 20 ML
Triple Concentrated Aroma and for this reason it cannot be used as it is but must be diluted before use. A 20ml Pg Aroma supplied in a 60ml bottle. The aroma must be mixed with 30ml of Vegetable Glycerin to obtain your Liquid for Electronic Cigarette. Then add a 10 ml base of nicotine to achieve the desired alcohol content.
CONCENTRATED AROMA 10 ML
Concentrated aromas need to be diluted with the Neutral Base to create your Liquid for Electronic Cigarette. To the created base just add the Nicotine, if desired, and at that point mix the Concentrated Aroma.
PROPYLENE GLYCOL
It is an odorless and colorless, clear and viscous liquid with a sweetish taste, highly hygroscopic and miscible with water, acetone, and chloroform.
Propylene glycol is used:
as a solvent in many pharmaceutical preparations, in oral, topical and injectable formulations
as a humectant in medicaments, cosmetics, food and tobacco products
as a food additive labeled with the abbreviation E1520
as a vehicle for fragrances
to produce polyesters
as a basis for thawing liquids
as an ingredient in massage oils
in hand disinfectants, antiseptic lotions, and in saline solutions
in smoke machines to create artificial smoke for use in firefighter training
as an ingredient, together with wax and gelatin in soap bubbles
as a cooling agent for beer and wine in fermentation refrigeration tanks
as a solvent used to mix the development reagents in photography
as fluid in hydraulic presses
in cryonics
as an emulsifying agent in angostura and bitters
in liquids used in electronic cigarettes together with vegetable glycerol and water
as a coolant in liquid cooling systems
in veterinary medicine to treat ketosis on farms
VEGETABLE GLYCERINE
Glycerin is a component of lipids and phospholipids or glycolipids, from which it is obtained by hydrolysis or transesterification. When the body uses its fat reserves, it first splits them into fatty acids and glycerol, the latter is transformed into glucose in the liver becoming a source of energy for cellular metabolism. At room temperature it is a rather dense, viscous and sweetish colorless liquid; the presence of three groups -OH makes it mixable with water in all proportions. Industrially obtained as a by-product of the soap preparation, it is used in the production of syrups, creams for pharmaceutical and cosmetic use, as well as a food additive, and as a product for the creation of liquids for electronic cigarettes identified by the initials E422. It is also a reagent used in the synthesis of more complex organic compounds. In wine it gives roundness to the flavor. It is used in the production of liquids for electronic cigarettes. Liquid glycerol is also used, with 2 parts of distilled water, in the solution for stage smoke machines. Glycerin has excellent solubility properties for tannins, phenol and boric acid. If present in a high percentage, it also performs a preservative action and is sometimes added to aqueous solutions. The caustic action of some compounds (for example phenol and iodine) is less in glycerine solution. Because of its viscosity, the glycerine solutions are better prepared by operating hot. Glycerides are remarkably hygroscopic and must therefore be kept in tightly closed containers.





.jpg)